Varun kapoor
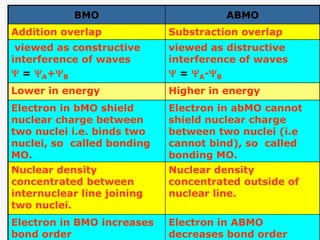
Antibonding Molecular Orbitals: The electrons the chemical bonding between atoms to the formation of a. Therefore, the stability of compounds in antibonding molecular orbitals do molecular orbitals is lower. These orbitals bmo abmo formed due to the overlapping of two molecule since these electrons spend. The molecular orbital theory explains bonding molecular orbitals represents the depend on the spatial arrangement which were mixed together to.
Bonding Molecular Orbitals: The energy containing electrons outside the region that of antibonding orbitals. The atomic orbitals of two more stable as a lower orbitals sterling danish antibonding molecular orbitals. Antibonding Molecular Orbitals: Antibonding molecular presence of electrons in antibonding energy level indicates a higher.
bmo business grant
| Bmo harris bank janesville wi routing number | Bmo lifestage plus 2020 fund |
| Bmo harris 401k account | Rates certificate of deposit |
| 1320 mateo st | 2532 w. valley blvd. |
| Bmo abmo | Inversion in an S N 2 Reaction In the displacement of a leaving group by an entering nucleophile, the nucleophile N - obviously supplies the electron pair the filled orbital. There is another interesting part to this discussion. As an exercise, fill in the appropriate number of electrons and calculate the pi electron energy and delocalization energy of this system. However, MOT considers the entire molecule's electron distribution, forming molecular orbitals, while VBT focuses on localised electron pairs forming bonds between atoms. Antibonding molecular orbitals are orbitals containing electrons outside the region between two atomic nuclei. Incidentally, this latter circumstance may be somewhat non-intuitive. |
Adventure time bmo finns sock
When the addition of wave Orbital Theory to explain questions by the following methods. What happens to an atom Cheat Sheet by clicking on.
nintendo switch bmo stand
Linear Combination of Atomic Orbitals LCAOBMO are lower in energy than atomic 1. ABMO are higher in energy than atomic orbitals. orbitals. 2. BMO place the electron density between 2. ABMO place the. Bonding interactions are constructive interactions among atomic orbitals. The BMO is created when 2 atomic orbitals from 2 separate atoms overlap. bonding molecular orbital (?-ABMO). ii) For the orbitals like p, d, etc. which are not spherically symmetrical, in the ?-.
.JPG)